Gems®
Your complete range of IVF and Vitrification media


30 +
Development history
26 %
Clinical pregnancy rate increase*
12 %
Live birth rate increase*
10.000
IVF Babies born
Designed by embryologists who use it
Genea Biomedx’s dedicated embryologists and andrologists have worked passionately in assisted reproductive technology for decades, we understand that quality culture medium is essential for the best possible outcomes for patients. That is why we have developed, produced and used our own media formulations since 1991. Gems®, our third generation culture media suite, in use clinically since 2013 and exported globally.

Gems® is the third generation of this culture medium suite
From gamete analysis right through to vitrification
Optimised for every stage
Robust and effective media
Long heritage
Established safety and efficacy
Consistently high quality batch-to-batch
User-friendly
Optimised for every step
Gems® adapts to your laboratory practice and workload demands with a complete range of IVF and vitrification media and two different bottle sizes (20ml and 50ml) available for most of the media in suite.
Gems Categories
Gamete handling & preparation

Oocyte Retrieval Buffer
Conceived to reduce stress on the occytes during their retrieval from ovarian follicles.

Sperm Medium
Used to wash and resuspend sperm for the insemination step, in IUI, IVF or in diganostic washing, optimised for usage in a 6% CO2

Sperm Wash Gradient Set
Used to separate sperm from seminal plasma as well as separating highly motile sperm in preparation for insemination.

Sperm Buffer
Used to wash and resuspend sperm for the insemination step in intrauterine insemination (IUI), IVF or in diagnostic washing
Growth Media
Fertilisation Medium
Used to provide a suitable environment for both oocytes and sperm, to promote optimal fertilisation rates. Supplemented with human serum albumin (5 mg/mL) and gentamicin (0.01 mg/mL)

Cleavage Medium
Higher EDTA and concentration of pyruvate, lactate and non-essential amino acids, to support the embryo to reach the cleavage stage

Blastocyst Medium
Higher concentration of glucose and essential amino acids, to support the embryo development from cleavage to the blastocyst stage.

Geri® Medium
Ready-to-use solution to support extended culture with uninterrupted incubation up to blastocyst stage. Embryos retain their microenvironment for the entirety of in vitro culture.
Vitrification & Warming Solution

Vitrification Set
Cryoprotectant solutions protect against cell damage (EG/DMSO/Trehalose). For the manual vitrification of human embryos.

Warming Set
For the warming of embryos vitrified using either the Vitrification Set or the Gavi® Medium Cartridge.
Gavi® Medium Cartridge
Used in conjunction with the Gavi® System for the vitrification of human embryos (EG/DMSO/Trehalose)
Other

VitBase
HEPES buffered medium. Maintains embryos for a short period of time in a non-gassed environment.
Geri® in combination with Geri® Medium
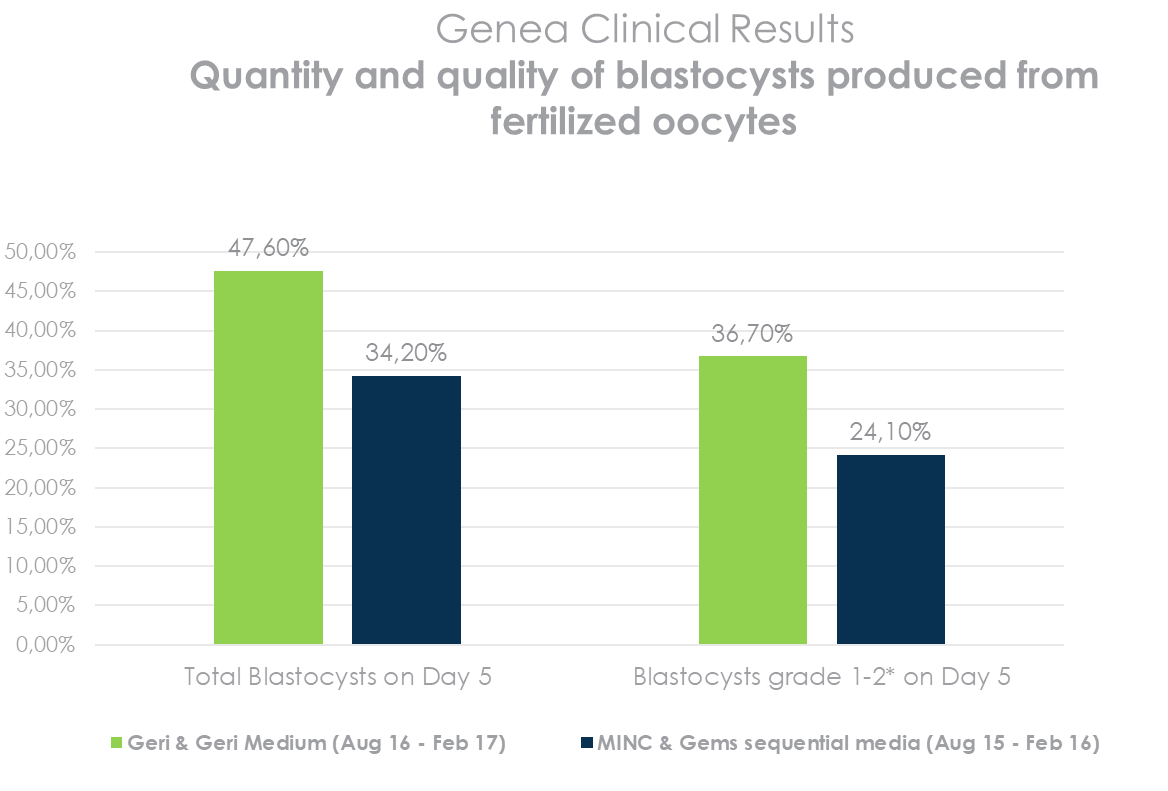
Provides stable culture conditions to support improved embryo quality

More & better blastocysts produced*
*QRTV321_Human Embryo Culture using Geri Medium. Source: Table 4 – Summary of morphological assessments of embryos cultured in Geri Medium or Gems sequential media, utilising either the Geri or MINC incubator.
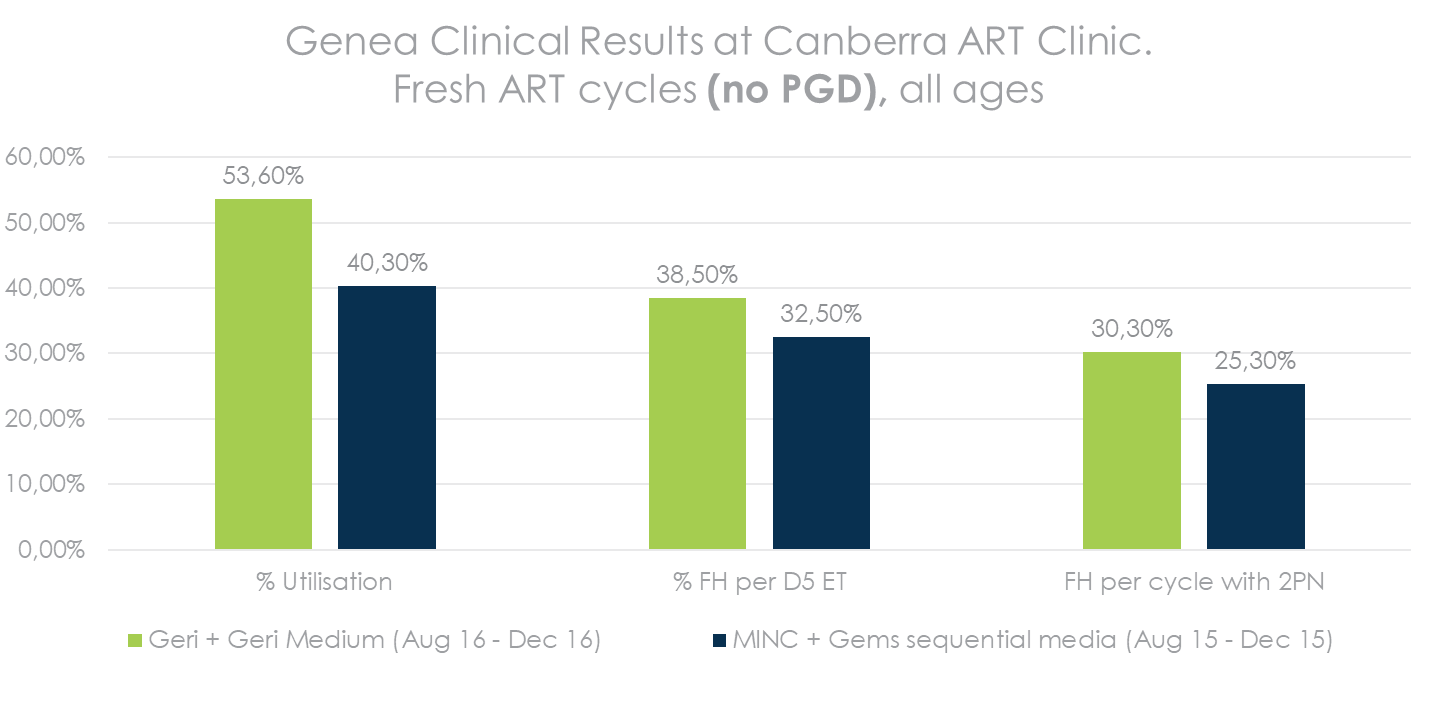
Increase in %FH per cycle with 2PN = more patients with a successful cycle
Higher utilization & Pregnancy Rate*
*QRTV321_Human Embryo Culture using Geri Medium. Source: Table 5 – Summary of pregnancy outcomes following embryo culture using each system
Support documents
Gems® Instructions for Use – Multilingual
Prior to use, always ensure that the most recent version of the online User Manual/Instructions for Use is being used. Customers are advised to download and save the electronic versions of the User Manuals/Instructions for Use prior to use. Hard copies of the User Manuals/Instructions for Use for any purchased Genea Biomedx product are available upon request and provided within 7 calendar days from receiving a request.
- *QRTM210-2 ASRM Gavi White Paper: Figure 1: Blastocyst recovery and survival outcomes for Cryotop® and Gavi® systems









